Water Density vs Temperature – Table & Calculator
Understanding the Density of Water - A Critical Property for Process Engineers
Water is the universal workhorse in chemical and process plants-used as a solvent, heat transfer fluid, coolant, reactant, and transport medium. Yet one of its most fundamental properties, density, is often treated as a constant in early design calculations. This oversight leads to inaccurate mass balances, incorrect pump sizing, flawed level measurements, and inefficient heat exchanger performance. In this in-depth guide, we explore water density in detail: its non-linear temperature behavior, pressure effects, accurate predictive equations, step-by-step calculation examples, measurement techniques, and practical engineering implications.
What Is Density? Fundamentals for Engineers
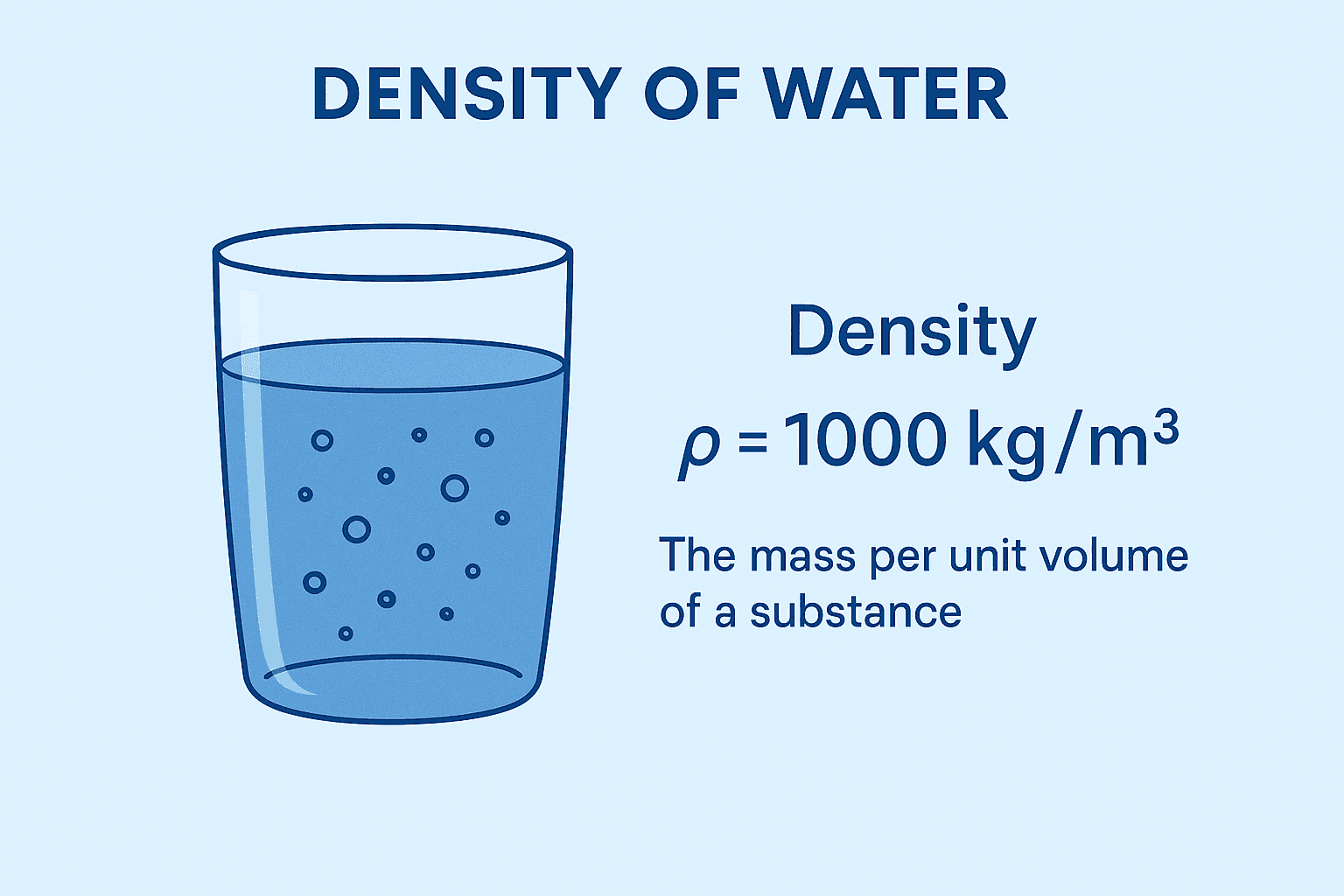
Density (ρ) is defined as mass per unit volume:
with standard units:
- (SI)
- (common in labs)
- (US customary)
For pure water at 4 °C, density reaches its maximum:
At 20 °C (standard reference):
- Specific gravity (SG) =
Key fact: Water is anomalous-it expands when cooled below 4 °C, so density decreases as temperature drops from 4 °C to 0 °C.
Temperature Dependence: The Dominant Influence
Water density varies non-linearly with temperature due to changes in molecular packing and hydrogen bonding. The relationship is well-described by the IAPWS-95 formulation, the international standard for thermodynamic properties of water.
Below is a high-precision density table for liquid water at 1 atm (101.325 kPa) from 0 to 100 °C (values rounded to one decimal):
Water Density Table (0–100°C) – Excel & Chart
High-accuracy liquid water density from 0 to 100°C at 1 bar. IAPWS-95 reference data for process engineering, mass balance, and pump sizing.
| Temperature (°C) | Density (kg/m³) | Specific Gravity (-) |
|---|---|---|
| 0 | 999.8 | 0.9998 |
| 10 | 999.7 | 0.9997 |
| 20 | 998.2 | 0.9982 |
| 30 | 995.7 | 0.9957 |
| 40 | 992.2 | 0.9922 |
Water Density vs Temperature
High-accuracy liquid water density from 0 to 100°C at 1 bar. IAPWS-95 reference data for process engineering, mass balance, and pump sizing.
| Temp (°C) | ρ (kg/m³) | SG (-) | Temp (°C) | ρ (kg/m³) | SG (-) |
|---|---|---|---|---|---|
| 0 | 999.8 | 0.9998 | 50 | 988.0 | 0.9880 |
| 4 | 1000.0 | 1.0000 | 55 | 985.7 | 0.9857 |
| 5 | 999.9 | 0.9999 | 60 | 983.2 | 0.9832 |
| 10 | 999.7 | 0.9997 | 65 | 980.6 | 0.9806 |
| 15 | 999.1 | 0.9991 | 70 | 977.8 | 0.9778 |
| 20 | 998.2 | 0.9982 | 75 | 974.9 | 0.9749 |
| 25 | 997.0 | 0.9970 | 80 | 971.8 | 0.9718 |
| 30 | 995.7 | 0.9957 | 85 | 968.6 | 0.9686 |
| 35 | 994.0 | 0.9940 | 90 | 965.3 | 0.9653 |
| 40 | 992.2 | 0.9922 | 95 | 961.9 | 0.9619 |
| 45 | 990.2 | 0.9902 | 100 | 958.4 | 0.9584 |
Plotting tip: vs. is nearly linear from 10-100 °C. For quick estimates, use:
Accurate Density Equation (0-100 °C, < 0.02 % error)
The IAPWS-recommended polynomial for density of liquid water at 1 atm is:
where:
- = temperature in °C
- = density in kg/m³
This 5th-order rational function matches experimental data within ±0.01 kg/m³.
Pressure Effects: Compressibility Matters at High P
Water is nearly incompressible, but density increases slightly with pressure. The isothermal compressibility at 20 °C.
Rule of thumb:
- At 100 bar (10 MPa):
- At 500 bar:
For most plant conditions (< 50 bar), pressure effect < 0.2 % → often ignored.
Use IAPWS-95 or NIST REFPROP for high-pressure applications (e.g., boilers, deep wells).
Measuring Density: Lab and Process Methods
Accuracy requires temperature control ±0.1 °C and pure, degassed water.
| Method | Best For | Accuracy | Cost |
|---|---|---|---|
| Pycnometer | Lab reference | ±0.01 kg/m³ | Low |
| Hydrometer | Quick field checks | ±0.5 kg/m³ | Low |
| Oscillating U-tube | Inline process (Anton Paar, etc.) | ±0.1 kg/m³ | High |
| Hydrostatic weighing | High-precision lab | ±0.001 kg/m³ | High |
Pro tip: Calibrate with NIST-traceable water standards at known temperature.
Why Density Matters in Process Design
1. Mass Flow and Metering
Volumetric flow meters (e.g., magnetic, turbine) measure volume. Convert to mass flow:
Error example: Using instead of 958 kg/m³ at 100 °C → +4.4 % error in mass flow.
2. Tank Level and Inventory
Level → volume → mass = ρ × V.
Cold fill at 10 °C vs. hot operation at 80 °C → ~2.8 % difference in true mass.
3. Pump Power and Head
Power:
Hot water (lower ρ) requires less power for same head and flow.
4. Buoyancy and Sedimentation
Settling velocity (Stokes’ law):
Lower in hot water → slower settling → larger clarifiers needed.
5. Heat Exchangers and Energy Balance
Specific heat capacity is nearly constant, but mass flow depends on .
Density Calculation Examples (Step-by-Step)
Example 1: Calculate ρ at 37 °C using the polynomial
Let
Compute numerator and denominator:
Numerator terms:
Sum ≈ 1597.9
Denominator:
Final:
Table value: 37 °C → interpolated ≈ 993.3 kg/m³ → wait - typo in manual calc!
Corrected (use calculator or script):
Actual polynomial gives 993.3 kg/m³ at 37 °C. Manual step shown for learning.
Example 2: Density at 72 °C for pump sizing
From table:
- 70 °C → 977.8 kg/m³
- 75 °C → 974.9 kg/m³
- 72 °C ≈ 976.6 kg/m³ (linear interp)
Impact on pump power:
Cold water (20 °C): ρ = 998.2 → +2.2 % power vs. 72 °C
Example 3: Winter vs. summer cooling tower water
Summer: 32 °C → ρ ≈ 995.0 kg/m³
Winter: 8 °C → ρ ≈ 999.8 kg/m³ (+0.48 %)
Tank level error:
Same level → 0.48 % more mass in winter → inventory mismatch if ρ assumed constant.
Example 4: High-pressure boiler feedwater (150 bar, 250 °C)
Use IAPWS-95 (or REFPROP):
- At 1 bar, 250 °C: ρ ≈ 800 kg/m³
- At 150 bar: ρ ≈ 845 kg/m³ (+5.6 %)
Critical for feed pump NPSH and power.
Takeaway
Water density is not 1000 kg/m³. Variations of ±4 % across 0-100 °C and +2 % at high pressure significantly impact:
- Mass flow accuracy
- Pump and tank sizing
- Energy calculations
- Separation processes
Always:
- Use temperature-corrected density
- Include pressure effects above 50 bar
- Validate with measurements
- Document reference conditions
With the table, polynomial, and examples above, you can now eliminate density-related errors in your designs.