Water Vapor Pressure vs Temperature – Table & NPSH
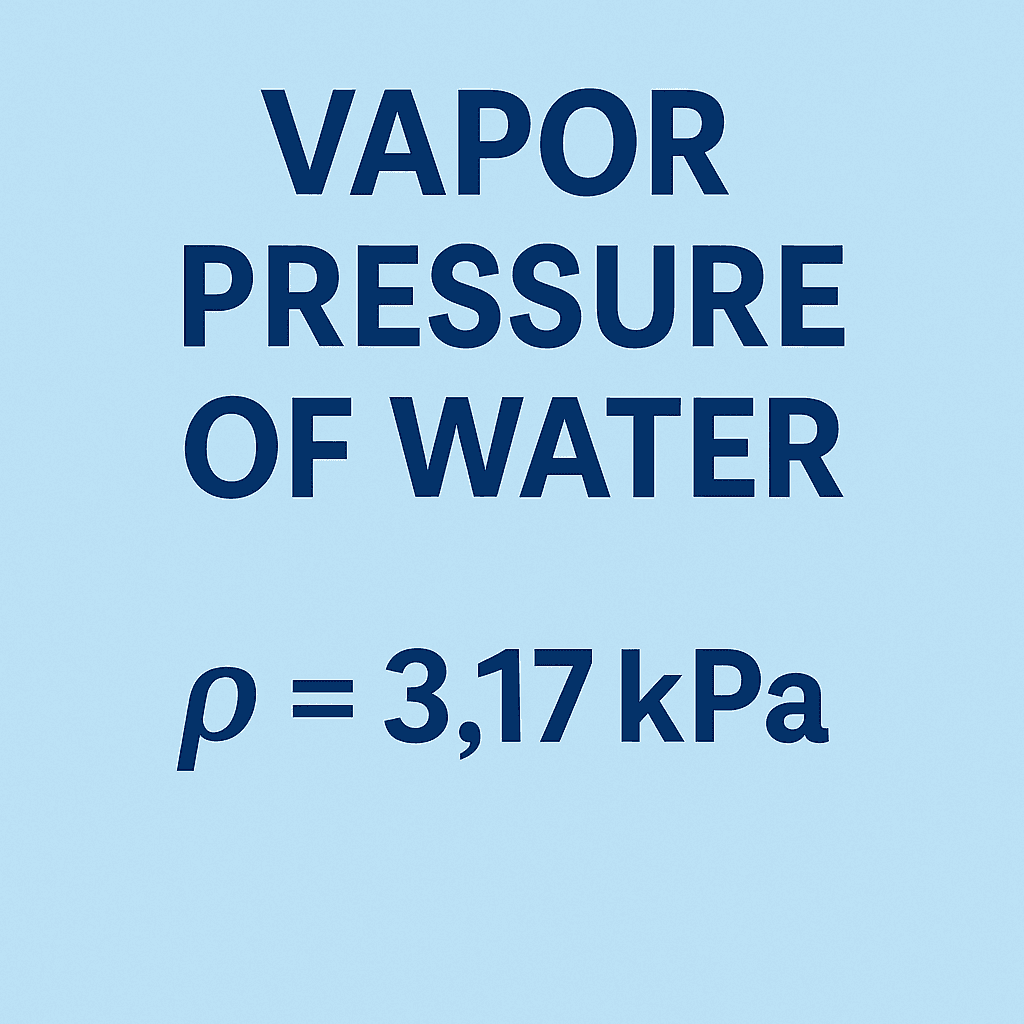
Vapor Pressure of Water - Predicting Boiling, Cavitation, and Flash in Pipes
Water doesn’t boil at 100 °C in every system. It flashes at 60 °C in a vacuum line, causes cavitation in a hot pump suction, or stays liquid at 150 °C under pressure. The key? Vapor pressure-the pressure at which liquid and vapor are in equilibrium.
Misjudging vapor pressure leads to pump failure, pipe hammer, flash explosions, undersized condensers, and lost production. This post delivers accurate vapor pressure data, IAPWS-approved equations, step-by-step calculations, NPSH examples, and plant-safe design rules.
What Is Vapor Pressure?
Vapor pressure (Pᵥ) is the pressure exerted by water molecules escaping from the liquid phase at a given temperature. At Pᵥ = P_system, the liquid boils.
Units:
- kPa, bar, mmHg, psia
- 1 atm = 101.325 kPa = 760 mmHg
For pure water:
- At 20 °C:
- At 100 °C: (boiling at 1 atm)
Critical point: 374 °C, 221 bar - vapor and liquid become indistinguishable.
Temperature Dependence: The Clausius-Clapeyron Effect
Vapor pressure rises exponentially with temperature due to increased molecular kinetic energy.
Below is a high-accuracy table from IAPWS-95 (rounded to 2 decimals):
Water Vapor Pressure vs Temperature (0–374°C)
Saturation vapor pressure of water. Essential for NPSH, flash, vacuum systems, and boiling point elevation.
| Temperature (°C) | Vapor Pressure (kPa) |
|---|---|
| 0 | 0.61 |
| 20 | 2.34 |
| 40 | 7.38 |
| 60 | 19.95 |
| 80 | 47.39 |
Water Vapor Pressure vs Temperature
Saturation vapor pressure of water. Essential for NPSH, flash, vacuum systems, and boiling point elevation.
| Temp (°C) | Pᵥ (kPa) | Pᵥ (mmHg) | Temp (°C) | Pᵥ (kPa) | Pᵥ (mmHg) |
|---|---|---|---|---|---|
| 0 | 0.61 | 4.58 | 50 | 12.35 | 92.6 |
| 5 | 0.87 | 6.54 | 60 | 19.95 | 149.6 |
| 10 | 1.23 | 9.21 | 70 | 31.19 | 233.9 |
| 15 | 1.71 | 12.8 | 80 | 47.39 | 355.4 |
| 20 | 2.34 | 17.5 | 90 | 70.14 | 526.0 |
| 25 | 3.17 | 23.8 | 100 | 101.33 | 760.0 |
| 30 | 4.25 | 31.8 | 120 | 198.5 | 1489 |
| 35 | 5.63 | 42.2 | 150 | 476.0 | 3570 |
| 40 | 7.38 | 55.3 | 200 | 1554 | 11650 |
| 45 | 9.59 | 71.9 | 250 | 3973 | 29790 |
Rule of thumb: Every 10 °C rise ≈ doubles Pᵥ below 100 °C.
Accurate Antoine Equation (1-100 °C, < 0.5 % error)
where:
- = vapor pressure in mmHg
- = temperature in °C
| Range | A | B | C |
|---|---|---|---|
| 1-100 °C | 5.11564 | 1687.537 | 230.17 |
To get kPa:
IAPWS-97 Equation (0-374 °C, < 0.1 %)
For high accuracy (used in REFPROP, Aspen, etc.):
- , ,
- Coefficients:
, , ,
, ,
Pressure Effects: Dissolved Gases and Salts
- Pure water only: Use above equations.
- Air-saturated water: Total pressure = Pᵥ + P_air → slightly higher boiling point.
- Brine (5% NaCl): Pᵥ drops ~20 % → higher boiling point.
Use Raoult’s law for dilute solutions.
Measuring Vapor Pressure
| Method | Best For | Accuracy | Cost |
|---|---|---|---|
| Static method (manometer) | Lab reference | ±0.1 % | Medium |
| Dynamic (boiling point) | Quick check | ±1 % | Low |
| Pressure transducer | Inline (vacuum systems) | ±0.5 % | High |
| IAPWS/REFPROP | Design & simulation | ±0.05 % | Free |
Why Vapor Pressure Matters in Process Design
1. Pump NPSH and Cavitation
NPSHa must exceed NPSHr + margin:
Hot water at 80 °C:
→ Only 54 kPa gauge available before cavitation.
2. Flash in Pipes and Valves
Pressure drop → P < Pᵥ → flash steam → shock, erosion, noise.
3. Vacuum Systems and Condensers
Condenser pressure = Pᵥ at cooling water T.
30 °C water → min vacuum ≈ 4.2 kPa abs.
4. Safety in Hot Water Loops
120 °C water at 1 bar → Pᵥ = 198 kPa → instant flash if line breaks.
5. Distillation and Evaporation
Bubble point = Pᵥ → controls column pressure.
Vapor Pressure Calculation Examples
Example 1: Antoine at 75 °C
Table: 70 °C = 31.2 kPa, 80 °C = 47.4 kPa → 75 °C ≈ 38.7 kPa
Error < 0.2 %
Example 2: Flash temperature in vacuum line
Water at 1 kPa abs → find boiling T.
From table:
- 0.61 kPa → 0 °C
- 1.23 kPa → 10 °C
Interpolate: ~7 °C
Any water >7 °C will flash.
Example 3: NPSHa for hot water pump
- Fluid: Water at 90 °C
- Suction: 1.5 m static head, 0.5 m friction loss
- Tank pressure: 1.2 bar gauge = 221.3 kPa abs
NPSHr = 3 m → Cavitation risk!
Fix: Increase tank pressure or cool suction.
Example 4: Condenser vacuum limit
Cooling water at 32 °C →
Minimum condenser pressure ≈ 5-6 kPa abs (with margin).
Lower → impossible without subcooling.
Takeaway
Vapor pressure controls:
- Boiling point
- Pump cavitation
- Flash risk
- Vacuum limits
Never assume 100 °C boiling. Use:
- Antoine for 1-100 °C
- IAPWS for high T/P
- Tables for daily checks
With the data and examples above, you can now size safe hot water systems, prevent pump damage, and optimize vacuum processes.