Why Water Viscosity Changes with Temperature
The Hidden Physics: Why Water Viscosity Changes with Temperature
The viscosity of water dramatically changes with temperature, dropping more than six times from 0°C to 100°C. While we often notice water feels "thinner" when hot, this physical property has profound implications in engineering and fluid mechanics. At 0°C, water has a dynamic viscosity of 0.0017914 Pa·s, but this decreases significantly to just 0.0002816 Pa·s at 100°C. :contentReference[oaicite:1]{index=1}
Water viscosity, essentially a measure of resistance to flow, plays a crucial role in determining how fluids behave in various applications. Understanding both dynamic viscosity of water (absolute viscosity) and kinematic viscosity of water helps engineers calculate the Reynolds number to determine if fluid flow is laminar, transitional, or turbulent.
Understanding Viscosity in Water
Viscosity represents a fluid's resistance to flow or deformation under applied force. For water, understanding viscosity provides crucial insights into its behavior across numerous applications.
Dynamic Viscosity vs Kinematic Viscosity of Water
Dynamic viscosity (μ) measures the fluid’s internal resistance to flow under shear stress.
Kinematic viscosity (ν) is defined as:
[ \nu = \frac{\mu}{\rho} ]
Where:
- μ = dynamic viscosity
- ρ = density
Two fluids can have the same dynamic viscosity but different kinematic viscosities due to density differences.
Units of Measurement
Dynamic Viscosity Units
- Pascal-second (Pa·s) - SI unit
- Centipoise (cP) - 1 cP = 0.001 Pa·s
- Poise (P) - 1 P = 0.1 Pa·s
- lb/(ft·h)
Water at 20.2°C = 1.0 cP
Kinematic Viscosity Units
- m²/s - SI unit
- Centistokes (cSt) - 1 cSt = 10⁻⁶ m²/s
- Stokes (St)
At 20°C, water ≈ 1.0038 cSt
Why Viscosity Matters in Fluid Mechanics
- Determines laminar vs turbulent flow
- Directly affects pressure drop
- Influences pump selection
- Governs heat transfer performance
- Controls lubrication effectiveness
How Temperature Affects Water Viscosity
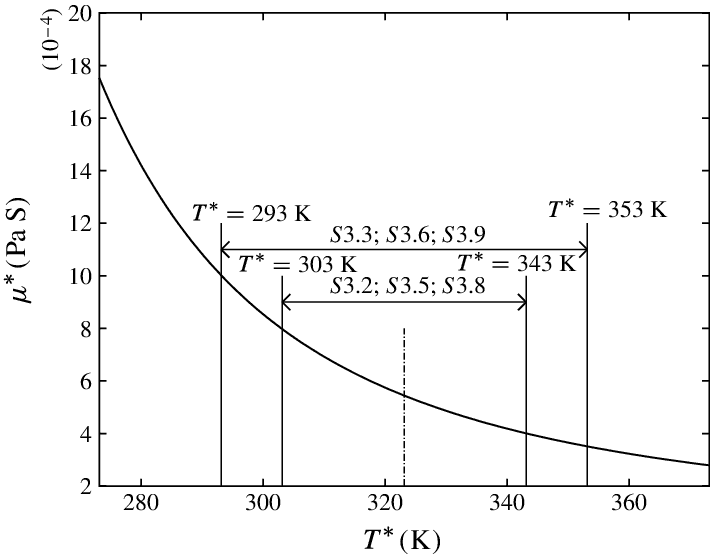
Water viscosity decreases exponentially with rising temperature:
| Temperature (°C) | Dynamic Viscosity (cP) |
|---|---|
| 0 | 1.7914 |
| 20 | ≈ 1.0016 |
| 50 | ≈ 0.547 |
| 80 | ≈ 0.355 |
| 100 | ≈ 0.2818 |
This represents a six-fold decrease from freezing to boiling point.
Kinematic Viscosity of Water at Different Temperatures
- At 0°C → ~1.7918 cSt
- At 20°C → ~1.0035 cSt
- At 100°C → ~0.2938 cSt
Pressure has a minor effect compared to temperature for most engineering conditions.
Viscosity Drop per 10°C Rise
- 0 → 10°C: ~27% decrease
- 10 → 20°C: ~23% decrease
- 20 → 30°C: ~20% decrease
- 40 → 50°C: ~16% decrease
- 90 → 100°C: ~10% decrease
The rate of drop slows at higher temperatures.
Molecular Interactions Behind Viscosity Changes
Hydrogen Bonding & Cohesion
Water molecules form extensive hydrogen bond networks, creating stronger resistance to motion.
Thermal Agitation
With higher temperature:
- Molecular velocity increases
- Hydrogen bonds weaken
- Intermolecular attraction decreases
- Flow resistance drops
Phase Structure Limits
- Maximum density at 4°C
- Viscosity collapse near 96°C
- Poor Arrhenius behavior - better predicted by Vogel-Fulcher models
Real-World Implications of Viscosity Variations
Effect on Reynolds Number
[ Re = \frac{vL}{\nu} ]
Lower viscosity means higher Reynolds number at the same flow rate → increased turbulence.
Viscosity in Heating & Cooling Systems
- Lower viscosity → better heat transfer
- Lower viscosity → lower pumping power
- High cold-start viscosity helps engines warm faster
Viscosity in Biological Systems
- Controls cilia beating
- Affects sperm mobility
- Influences microbial swimming
- Determines filtration rates
Unit Conversion Reference
Dynamic Viscosity
- 1 cP = 0.001 Pa·s
- 1 Pa·s = 1000 cP
- 1 cP = 2.419 lb/(ft·h)
Kinematic Viscosity
- 1 cSt = 10⁻⁶ m²/s
- 1 cSt = 1.076 × 10⁻⁵ ft²/s
Using Viscosity Tables in Practice
- Identify if μ or ν is required
- Note operating temperature
- Convert into required engineering units
Example at 20°C:
- Dynamic viscosity ≈ 1.0016 mPa·s
- Kinematic viscosity ≈ 1.0034 mm²/s
Conclusion
Water viscosity is a temperature-dependent property controlled by molecular hydrogen bonding. From 1.79 cP at 0°C to 0.28 cP at 100°C, this dramatic reduction governs:
- Flow regimes
- Heat transfer efficiency
- Pumping power
- Biological mobility
- Chemical transport processes
This microscopic physics explains why hot water flows more easily than cold water and forms the foundation of precise engineering system design.
Key Takeaways
- Water viscosity drops six-fold between 0°C and 100°C
- Hydrogen bonding governs viscosity behavior
- Reynolds number changes directly with viscosity
- Higher temperatures reduce pumping energy
- Viscosity strongly controls biological transport
- Engineers must handle proper unit conversions
FAQs
Q1. How does temperature affect water viscosity?
It decreases viscosity by weakening hydrogen bonds, allowing molecules to move more freely.
Q2. Difference between dynamic and kinematic viscosity?
Dynamic measures internal resistance, kinematic includes density effects.
Q3. Why is viscosity critical in engineering?
It controls flow regime, heat transfer, pressure loss, and pump sizing.
Q4. How does viscosity affect biology?
It governs microorganism motion, filtration, and cellular transport.
Q5. Can viscosity be changed without temperature?
Yes-using additives or experimental fluid substitutes.